Our current research focus primarily stems from following three major topics:
- The role of the hypothalamus as a control center of aging in mammals
- The role of systemic NAD+ metabolism in mammalian aging and longevity control
- Human clinical trials on NMN (in collaboration with Dr. Samuel Klein’s group)
1. The role of the hypothalamus as a control center of aging in mammals
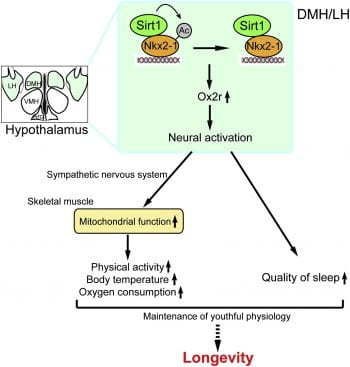
In the mammalian brain, the hypothalamus is a critical regulator for many physiological functions, including feeding behavior, endocrine regulation, circadian rhythms, and emotion. Our lab has previously shown that in the dorsomedial and lateral hypothalamic nuclei (DMH and LH, respectively), the mammalian NAD+-dependent deacetylase SIRT1 and its binding partner NKX2-1 cooperate to counteract age-associated physiological decline and promote longevity in mice (Satoh et al., Cell Metab., 2013). Additionally, skeletal muscle has been identified as a key effector that receives stimulation from the hypothalamus through the sympathetic nervous system and likely regulates other tissue functions (Satoh et al., Cell Metab., 2013; Snyder-Warwick et al., Aging Cell, 2018).
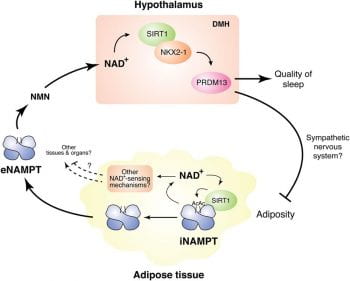
Because SIRT1/NKX2-1-double positive neurons in the DMH and LH are critical to counteract age-associate physiological decline, we are now trying to identify and characterize key neuronal subpopulations in the DMH and LH that may play a critical role in mammalian aging and longevity control. Prdm13-positive neurons have been characterized as one such neuronal subpopulation (Satoh et al., Aging Cell, 2015). To elucidate the function of Prdm13-positive neurons, we are collaborating with Dr. Akiko Satoh, a former Imai lab member and now a PI at the National Center for Geriatrics and Gerontology in Japan. We are also investigating the role of other previously uncharacterized DMH neuronal subpopulations in mammalian aging and longevity control. We are planning to manipulate the functions of these neuronal subpopulations by optogenetics and/or DREADDs and examine aging phenotypes and lifespan in mice.
We are also interested in the importance of other brain regions, such as the hippocampus, in age-associated cognitive changes. For example, we have demonstrated that age-related deficits in hippocampal NAD+biosynthesis is important for aging-associated cognitive dysfunctions (Stein et al., J. Neurosci, 2014; Johnson et al., NPJ Aging Mech Dis, 2018) and also for the age-associated depletion of the hippocampal neural stem cell pool (Stein et al., EMBO J., 2014). We are continuing to investigate the importance of NAD+ metabolism in age-associated cognitive dysfunctions.
As a new direction in the lab, we are now exploring age-associated changes in behavioral responses to social isolation. Social isolation is a serious problem for an increasing number of elderly. By using a social isolation paradigm in mice, we are trying to identify age-associated changes in neuronal circuits and molecular mechanisms that affect behavioral phenotypes in aged individuals.
2. The role of systemic NAD+ metabolism in mammalian aging and longevity control
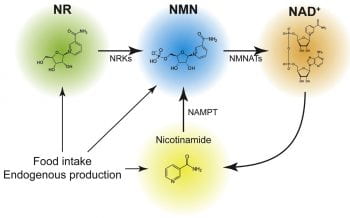
NAD+ is well known as a ubiquitous coenzyme critical for metabolic redox reactions. Importantly, it is also a substrate for many enzymatic activities, including those of poly-ADP-ribose polymerases (PARPs), sirtuins, and CD38/CD157 ectoenzymes. Recently, It has been established that during aging, the level of NAD+ decreases systemically, leading to many age-associated functional changes (Imai and Guarente, NPJ Aging Mech Dis, 2016; Yoshino, Baur and Imai, Cell Metab., 2018). Furthermore, boosting NAD+ biosynthesis by supplementing with NAD+ intermediates such as NMN and nicotinamide riboside (NR) in vivo results in a variety of anti-aging effects.
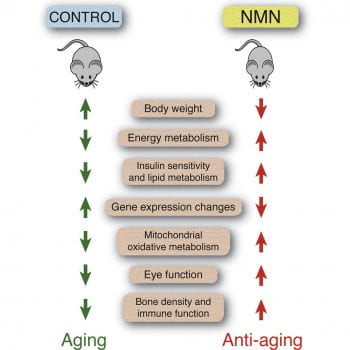
We have demonstrated that long-term NMN administration in mice conveys remarkable anti-aging effects, including the suppression of body weight gain, the improvement of insulin sensitivity and oxidative metabolism, and the enhancement of physical activity, eye function, bone density, and myeloid-lymphoid composition (Mills et al., Cell Metab, 2016). NMN also significantly increases the neural stem cell pool in the hippocampus (Stein et al., EMBO J., 2014) and improves cognitive hypersensitivity in aged mice (Johnson et al., NPJ Aging Mech Dis, 2018).
Recently, we have identified SLC12A8 as a novel NMN-specific transporter in mammals, which is highly expressed in the small intestine and plays an important role in maintaining systemic NAD+ homeostasis during aging (Grozio et al., Nat Metab, 2019). We are currently investigating the functions of SLC12A8 in different tissues, including the hypothalamus, in the regulation of metabolism and aging.
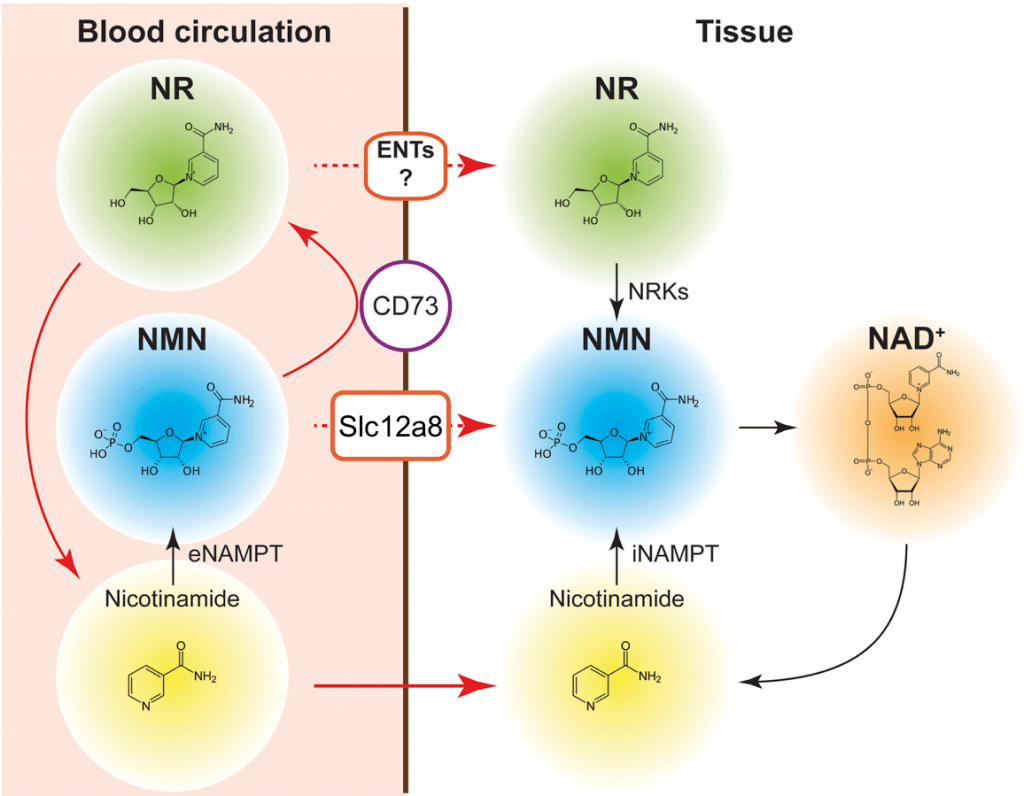
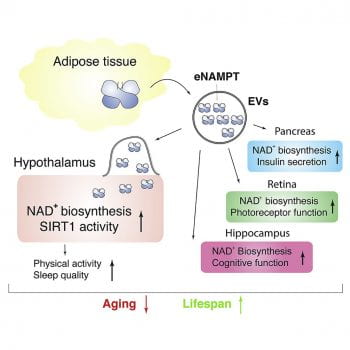
Another important focus is NAMPT, the rate-limiting enzyme for the major NAD+ biosynthetic pathway in mammals. NAMPT-mediated NAD+ biosynthesis is important for the regulation of SIRT1 activity (Revollo, Grimm, and Imai, JBC, 2004). We have previously shown that mammalian NAMPT exists in two forms: intra- and extracellular NAMPT (iNAMPT and eNAMPT, respectively) (Revollo et al., Cell Metab, 2007). In adipose tissue, SIRT1 deacetylates iNAMPT to predispose it to secretion as eNAMPT. This adipose secretion pathway accounts for a large portion of circulating eNAMPT (Yoon et al. Cell Metab, 2015). Interestingly, eNAMPT is critical to maintain hypothalamic NAD+ biosynthesis, suggesting a novel inter-tissue communication between adipose tissue and the hypothalamus (Yoon et al. Cell Metab, 2015). Both iNAMPT and eNAMPT levels decline with age, contributing to the age-associated systemic decline in NAD+ levels (Yoshino et al., Cell Metab, 2011; Yoshida et al., Cell Metab, 2019). Most recently, we have found that circulating eNAMPT is carried in extracellular vesicles (EVs) and delivered to specific tissues, promoting NAD+ biosynthesis in target cells (Yoshida et al., Cell Metab, 2019). Genetically engineered mice that can maintain higher levels of circulating eNAMPT over age showed remarkable anti-aging phenotypes and a significant extension of healthspan. Furthermore, old mice supplemented with EVs purified from young mice showed enhanced physical activity and extended lifespan (Yoshida et al., Cell Metab, 2019). These findings have clearly demonstrated that eNAMPT plays a critical role in mammalian aging and longevity control. These studies also highlight adipose tissue as a key modulator of hypothalamic function in the concept of the NAD World.
Through these projects, we aim to understand the inter-tissue communication between the hypothalamus, skeletal muscle, and adipose tissue in mammalian aging and longevity control. The anticipated outcome of these studies will allow us to develop effective anti-aging interventions to achieve “productive aging”.
3. Human clinical trials on NMN (in collaboration with Dr. Samuel Klein’s group)
Supported by all these important preclinical results, we are now conducting a human clinical trial on NMN (NCT031512389, “Effect of Nicotinamide Mononucleotide (NMN) on Cardiometabolic Function”). We are also going to launch a second clinical trial on NMN soon.